Determine the theoretical yield of pbcl2 . – Delving into the realm of chemistry, we embark on a journey to determine the theoretical yield of PbCl2, a compound with significant industrial applications. This exploration will delve into the intricacies of stoichiometry, chemical equations, and the factors that influence the actual yield, providing a comprehensive understanding of this fundamental concept.
Understanding the theoretical yield of PbCl2 is paramount in optimizing production processes and ensuring efficient utilization of resources. This guide will equip you with the knowledge and tools to accurately calculate the theoretical yield, enabling informed decision-making and maximizing productivity.
Introduction

The theoretical yield of PbCl2 is the maximum amount of PbCl2 that can be produced from a given amount of reactants, based on the stoichiometry of the reaction. Determining the theoretical yield is crucial in chemical reactions as it provides a benchmark against which the actual yield can be compared, allowing for the optimization of reaction conditions and the efficient use of resources.
Chemical Equation
The balanced chemical equation for the reaction to produce PbCl2 is:
Pb(s) + 2 HCl(aq) → PbCl2(aq) + H2(g)
In this reaction, lead (Pb) reacts with hydrochloric acid (HCl) to form lead chloride (PbCl2) and hydrogen gas (H2).
Stoichiometry
From the balanced chemical equation, the mole ratio between Pb and PbCl2 is 1:1. This means that for every mole of Pb that reacts, one mole of PbCl2 is produced. To determine the limiting reactant, we compare the number of moles of each reactant to the stoichiometric ratio.
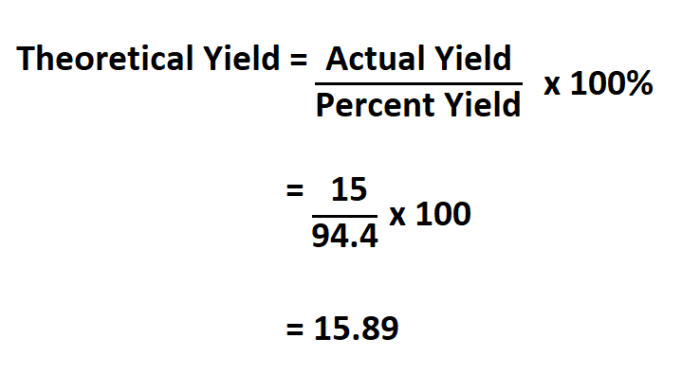
Calculations, Determine the theoretical yield of pbcl2 .
To calculate the theoretical yield of PbCl2, we use the following steps:
- Convert the mass of each reactant to moles using their respective molar masses.
- Compare the moles of each reactant to the stoichiometric ratio to determine the limiting reactant.
- Calculate the moles of PbCl2 that can be produced from the limiting reactant.
- Convert the moles of PbCl2 to grams using its molar mass.
For example, if we have 10.0 g of Pb and 20.0 g of HCl, we would first convert them to moles:
- Moles of Pb = 10.0 g / 207.2 g/mol = 0.0483 mol
- Moles of HCl = 20.0 g / 36.46 g/mol = 0.549 mol
Comparing the moles of each reactant to the stoichiometric ratio (1:2), we find that Pb is the limiting reactant because it has the smallest mole ratio (0.0483 mol / 2 = 0.0242). Therefore, the theoretical yield of PbCl2 is limited by the amount of Pb available.
To calculate the theoretical yield, we use the mole ratio between Pb and PbCl2 (1:1):
- Moles of PbCl2 = 0.0483 mol Pb × (1 mol PbCl2 / 1 mol Pb) = 0.0483 mol PbCl2
Finally, we convert the moles of PbCl2 to grams:
- Mass of PbCl2 = 0.0483 mol PbCl2 × 278.1 g/mol = 13.4 g
Therefore, the theoretical yield of PbCl2 is 13.4 g.
Factors Affecting Yield
Several factors can affect the actual yield of PbCl2, including:
- Purity of reactants
- Reaction conditions (temperature, pressure, time)
- Presence of impurities or side reactions
- Losses during the reaction or purification process
These factors can influence the theoretical yield by altering the stoichiometry of the reaction or by causing the formation of byproducts that reduce the amount of PbCl2 produced.
Applications
PbCl2 has various practical applications, including:
- Production of lead-based pigments for paints and ceramics
- Manufacturing of lead-acid batteries
- Electroplating of lead onto other metals
- Preparation of other lead compounds, such as lead acetate and lead nitrate
In these applications, the theoretical yield is crucial for optimizing production processes and ensuring the efficient use of resources.
Essential Questionnaire: Determine The Theoretical Yield Of Pbcl2 .
What is the significance of determining the theoretical yield of PbCl2?
Determining the theoretical yield provides a benchmark against which the actual yield can be compared, allowing for optimization of production processes and identification of areas for improvement.
How does stoichiometry play a role in calculating the theoretical yield?
Stoichiometry establishes the mole ratios between reactants and products, enabling the precise determination of the maximum amount of PbCl2 that can be produced from a given quantity of reactants.
What factors can affect the actual yield of PbCl2?
Factors such as side reactions, incomplete reactions, and impurities can influence the actual yield, resulting in a deviation from the theoretical yield.